Neuralink is getting ready to carry out a US clinical trial on the process of turning imagined speech into text, in a key step towards being able to communicate with just our minds for those who can no longer talk.
The study has had an investigational device exemption from the US Food and Drug Administration, which paves the way for its thought-to-text system to be tested in volunteers, company executives say.
And the promise is tantalizing: Read a person’s brain waves before that “I can’t go on” becomes a suicidal impulse, or when “I know what he really thinks of me” slips into full-blown paranoia. The dream doesn’t stop there — record visuals from our dreams so we may watch the same scene play out while we’re awake. It extends beyond what the company has shown off so far in terms of cursor control and goes for direct language decoding — arguably the most tangible benchmark for reestablishing communication.
What the Study Will Investigate in the October Trial
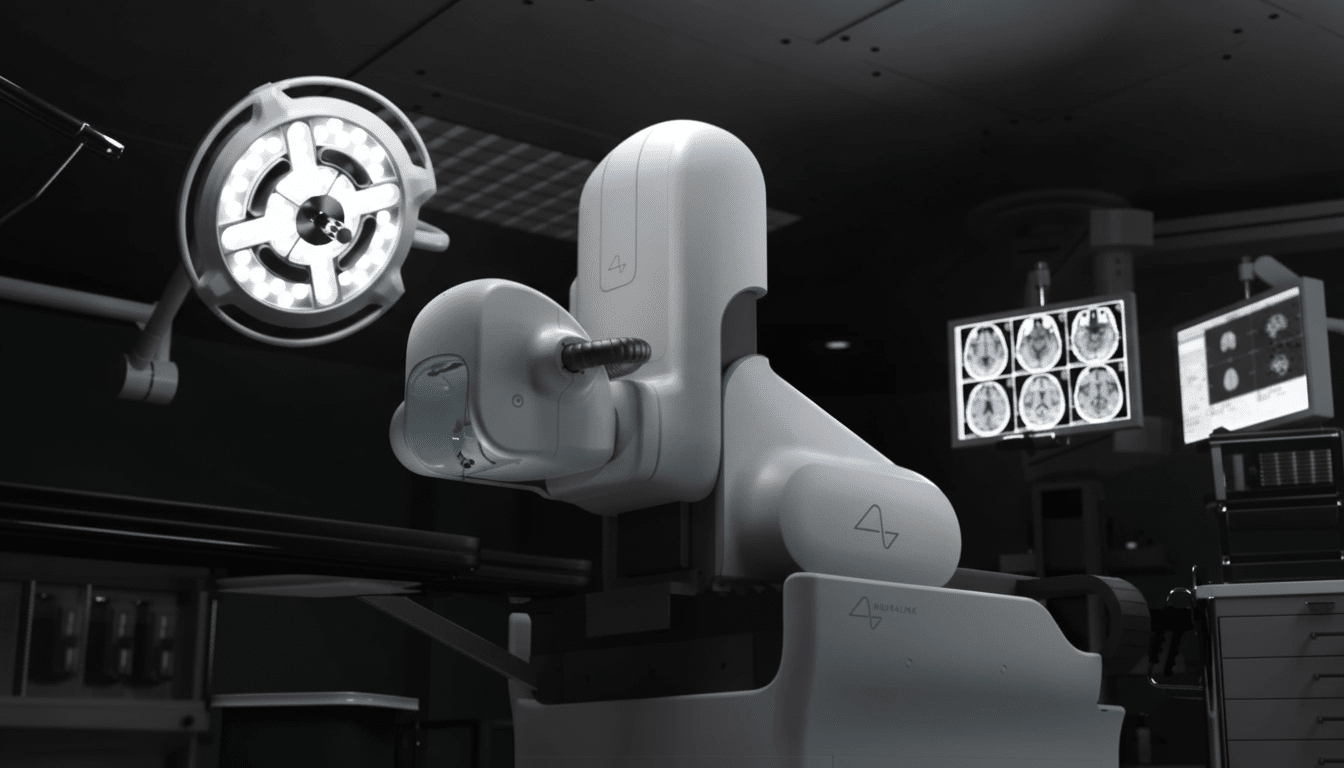
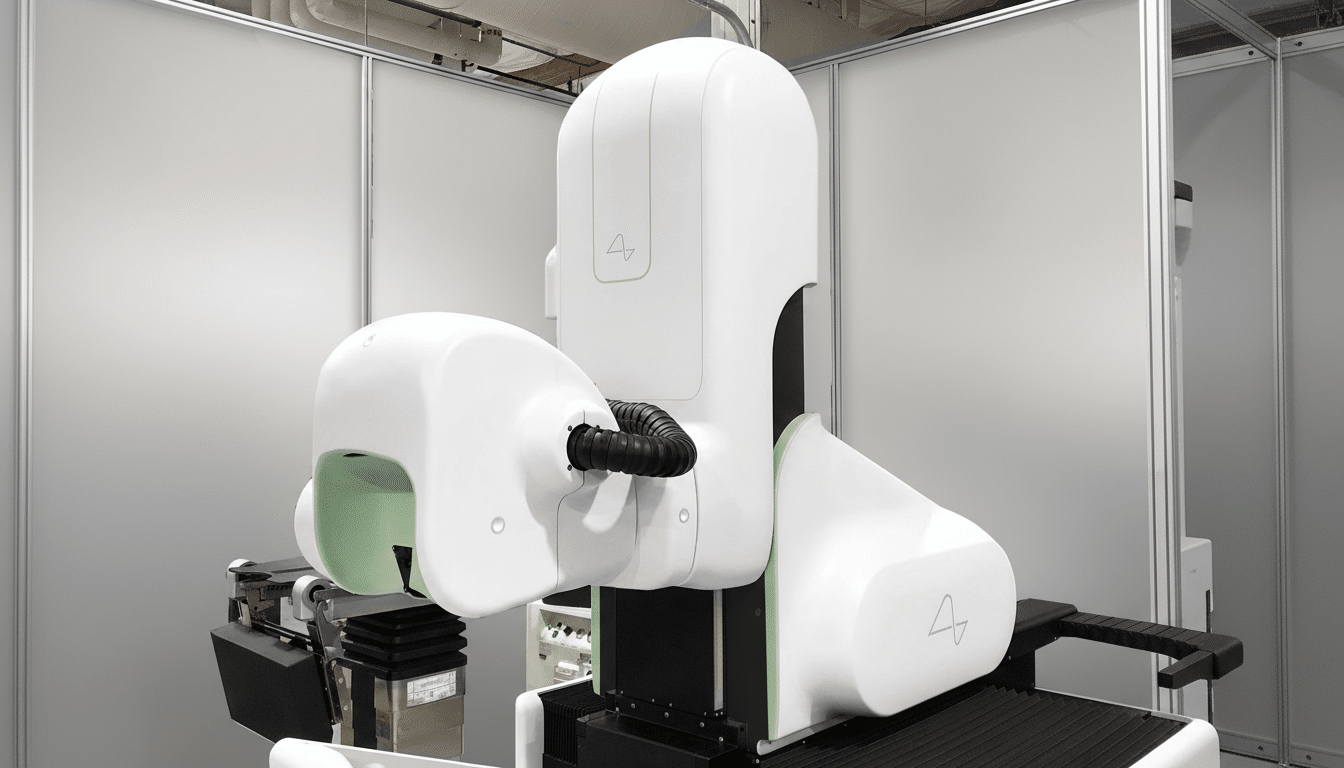
The case revolves around Neuralink’s N1 implant; it is a fully implanted brain-computer interface (BCI) with flexible threads and up to hundreds of recording channels that is inserted into the skull by the company’s R1 surgical robot. Practically speaking, participants will teach the system by trying to speak silently, as machine-learning models learn how to map patterns of neural activity onto letters, words and sentences. The primary end points are likely to be words per minute, word error rate and calibration time for home use of the decoder.
Neuralink has claimed that an increasing group of users is already walking around with the device, and the company recently suggested a dozen individuals now sport an N1 implant. Previous demonstrations showed messaging, email, and game pointer controls. The thought-to-text project hopes to shorten that process and make it more natural and language-first. Executives have also put out a “closed-loop” idea where large language models predict and then adjust the user’s intended text, and read it back to the person using everyday earbuds.
Regulatory Pathway and Participants for the Trial
An FDA investigational device exemption (IDE) permits experimental devices to be tested in humans, but it is not market clearance. First-line recipients are usually severely paralyzed patients, such as those with amyotrophic lateral sclerosis or spinal cord injury, for whom the benefit-risk balance tips favorably toward therapy. Safety-related endpoints will be established with respect to infection rate, seizure hazard, MRI compatibility and device longevity in conjunction with the performance criteria.
Neuralink has sketched out a distant vision that would bring its technology to consumers, even those who lack a medical need for the implants. That would involve numerous clinical phases, strong evidence across different populations, and FDA approval—likely through a premarket approval pathway. Their long-term reliability, strategies for revision surgery and guarantees of cybersecurity would all come under scrutiny before any widespread rollout.
How It Compares With Other BCIs and Academic Research
Academic groups have led the way in developing speech BCIs. Studies conducted by UCSF and Stanford researchers published in Nature described the potential for invasive arrays over speech motor areas to decode attempted speech at conversational rates, with word error rates that continued to improve as training progressed. Similarly, the BrainGate consortium also demonstrated high-accuracy text typing with intracortical recordings and language models. These findings show what could be achieved under controlled conditions — while making clear how difficult it is to apply that performance out in the wild.
Noninvasive methods including magnetoencephalography and functional MRI have also decoded language intent in laboratory settings, but they are dependent on large, costly equipment and aren’t yet practical for daily use. The results were mixed, but on the implant side, Synchron’s endovascular “stentrode” has allowed at-home texting and email in people with paralysis through a less invasive vascular route than that used by Neuralink, although it had five orders of magnitude lower bandwidth. Neuralink’s approach will be closely watched for evidence that it can pair high data rates with actual, practical real-world use.
The Hard Problems Ahead for Thought-to-Text Systems
Deciphering inner speech is a more challenging task than moving a cursor. Neural signals can drift over time, electrodes may move by micrometers and brain tissue might respond to implants. Neuralink has admitted early hardware problems — for instance, the movement of certain electrode threads — and suggested that subsequent software updates had improved its decoding. What patients and their doctors care about is whether they can keep stable performance for months, without recalibration every day.
There’s also a human factors layer: training fatigue, the cognitive load of silently “speaking,” and how aggressively to lean on language models. Predictive text can increase speed, but it also threatens to drive users toward the phrases they never intended. Privacy is another frontier. Neural data, along with strong models, is sensitive; clear policies around data ownership, encryption and consent will likely be striking targets for regulatory activity or ethical boards that have been enlightened by NIH BRAIN Initiative guidelines.
What Success Would Look Like for Neuralink’s Trial
In the short term, success will be outpacing the assistive communication tools of today. Current state-of-the-art eye tracking input methods typically deliver typing rates between low-to-mid teens wpm for expert users. If Neuralink’s system can perform well, stably and at home, rivaling or surpassing the performance of this control method — all while controllable error rates are kept in check with autocorrect — that would be a substantial clinical success for people living with ALS or high cervical spinal cord injuries.
Longer term, Neuralink imagines “closed-loop” interactions in which users scroll through apps, control objects and query large language models without using their voice or hands. That future depends on the results of the October trial, the company’s ability to scale safely and on how the FDA parses real-world benefit. For now, the study is a necessary test case: Can thought-to-text transfer from laboratory curiosities to reliable daily communication?

